Rutherford Atomic Model
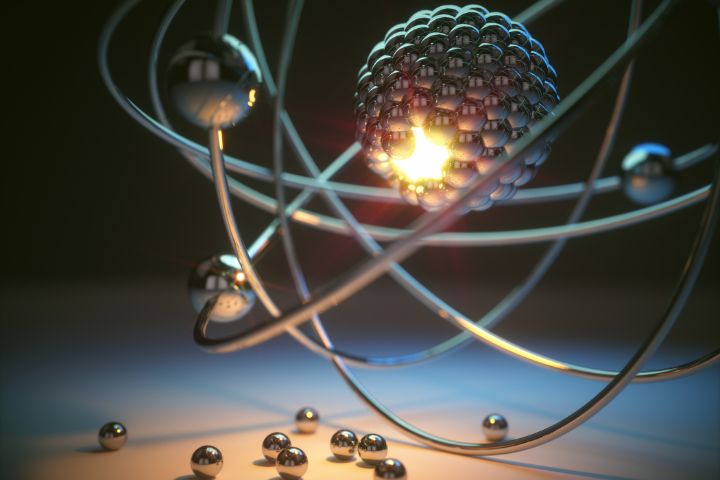
Rutherford's atomic model, proposed in 1911, is based on the existence of a central nucleus with a positive charge in the atom, where most of the mass is concentrated.
Today we will talk about
Electrons, with a negative charge, revolve around the nucleus in orbits. This model was developed from the famous gold foil experiment, where deviations in the trajectory of alpha particles were observed when bombarding gold foils.
Although the model had limitations, it was widely accepted and allowed advances in the study of the atom and its properties. Rutherford also made important contributions to physics and chemistry, such as the discovery of radioactive particles and the identification of the nucleus and proton.
What is Rutherford's atomic model?
The Rutherford atomic model, proposed by scientist Ernest Rutherford in 1911, revolutionized our understanding of the structure of the atom. This model is based on the existence of a central nucleus with a positive charge in the atom, in which most of the mass is concentrated. Electrons, with a negative charge, revolve around the nucleus in orbits.
Structure of the atom according to the Rutherford model
According to Rutherford's model, the atom is composed of a positively charged central nucleus, in which protons and neutrons are found, which concentrate most of the mass of the atom.
Electrons, with a negative charge, are in orbits around the nucleus. This image of the atom as a planetary system, with the nucleus like the sun and the electrons like the planets, was one of the main characteristics of Rutherford's model.
Distribution of charges in the atom according to the Rutherford model
According to Rutherford's model, the positive charge is concentrated in the nucleus, while the electrons revolve around it in orbits.
This distribution of charges in the atom explains the stability of the atom, since the attraction of the positive charges of the nucleus towards the electrons counteracts the electrostatic repulsion between the electrons. Furthermore, Rutherford's model postulated that the majority of the atom is empty space, since most of the alpha particles used in the gold leaf experiment passed through the atoms without deflecting, indicating the existence of a dense and positively charged nucleus in the atom.
Rutherford's contribution to the study of the atom
Ernest Rutherford is recognized as the father of nuclear physics and his experiments, including the famous gold leaf experiment, provided experimental evidence supporting his atomic model.
Additionally, Rutherford discovered radioactive alpha and beta particles, formulated the laws of radioactive decay, and was the first to identify the atomic nucleus and the proton. His work revolutionized our understanding of the structure of the atom and laid the foundation for future advances in physics and chemistry.
The gold leaf experiment and its results
The gold leaf experiment, carried out by Rutherford, was a fundamental step in the development of the atomic model. The experiment carried out and the results observed are described in detail below:
Description of the experiment carried out by Rutherford
Rutherford and his colleagues, Hans Geiger and Ernest Marsden, carried out the famous gold leaf experiment in their laboratory. It consisted of bombarding extremely thin sheets of gold with alpha particles, which are positively charged helium nuclei.
To carry out the experiment, the scientists used a source of alpha particles that emitted a constant stream of these particles. The alpha particles were directed towards the gold foil and their deflection was observed when interacting with the gold atoms.
Results observed in the gold leaf experiment
The results of the gold leaf experiment were surprising and defied scientists' expectations. Most of the alpha particles passed through the gold atoms without any deflection, indicating that the atom is mostly empty space.
On the other hand, some alpha particles were deflected or bounced at unexpected angles when interacting with the gold atom. These unexpected results led Rutherford to the conclusion that there was a dense, positively charged nucleus at the center of the atom, and that most of the mass was concentrated in this nucleus.
This finding was revolutionary, since it contradicted the previous atomic model, known as the Thomson model, which postulated a uniform atom with a homogeneously distributed positive charge.
Limitations of Rutherford's atomic model
The atomic model proposed by Rutherford failed to fully explain the stability of the atom and the distribution of electrons around the nucleus. The main limitations of this model are listed below:
Problems of stability and electronic distribution in the atom
Rutherford's model states that electrons revolve around the nucleus in defined orbits. However, according to the laws of classical physics, electrons should radiate energy as they spin and eventually collapse into the nucleus. This would mean a loss of stability and contradicts the observation that atoms are stable systems.
Another limitation of the model is the lack of a clear explanation of how electrons are distributed in different orbits around the nucleus. It does not provide information about the number of electrons that can occupy each energy level and how they are arranged in the electronic shells.
Later advances to overcome the limitations of Rutherford's model
After Rutherford's model, new theories were proposed that attempted to overcome these limitations. The main contributions were:
- Bohr model: In 1913, Niels Bohr proposed a model that included quantized energy levels. According to this model, electrons can only occupy specific, stable orbits around the nucleus, and jumps between orbits emit or absorb energy in the form of electromagnetic radiation.
- Quantum mechanics: With the development of quantum theory in the 1920s, new concepts were introduced that allowed us to better understand the electronic structure of atoms. Quantum mechanics describes electrons as both particles and waves, and provides a more precise description of the electronic distribution and stability of the atom.
These advances in the understanding of the atom made it possible to overcome the initial limitations of Rutherford's model and laid the foundations for later atomic models. For more information you can visit our article on the current atomic model.
Rutherford's contributions to physics and chemistry
Rutherford, recognized as the father of nuclear physics, made significant contributions to the study of the atom and its properties. His research into radioactivity and his gold leaf experiment laid the foundation for understanding atomic structure. Below are some of Rutherford's major contributions to the field of physics and chemistry.
Discovery of radioactive alpha and beta particles
During his research, Rutherford identified two types of radioactive particles: alpha particles and beta particles. Alpha particles consist of positively charged helium nuclei, while beta particles are high-energy electrons. This discovery revealed the existence of subatomic particles within the atom and marked an important milestone in the development of the atomic model.
Formulation of the laws of radioactive decay
Rutherford was a pioneer in the study of radioactive decay, establishing the fundamental laws that govern this phenomenon. His research demonstrated that radioactive particles decay over time in a predictable and constant manner. These laws, known as Rutherford's laws, laid the foundation for the later development of nuclear physics and radiometric dating.
Identification of the atomic nucleus and proton
Perhaps one of Rutherford's most notable contributions was the identification of the atomic nucleus. Through his famous gold leaf experiment, he observed that most alpha particles passed through the atom without deflecting significantly, which led to the conclusion that most of the mass of the atom is concentrated in a dense, positively charged core.
Furthermore, the existence of a subatomic particle that would be responsible for carrying this positive charge was postulated, which Rutherford called "proton." This discovery marked an important advance in the understanding of the structure and electrical charge of the atom, laying the foundation for the subsequent development of the atomic model.
In summary, Rutherford made relevant contributions to physics and chemistry, among which the discovery of radioactive alpha and beta particles, the formulation of the laws of radioactive decay, and the identification of the atomic nucleus and the proton stand out. These investigations laid the foundations for the development of later theories and allowed a greater understanding of the atomic structure and its properties.
Once these subatomic particles were discovered and the forces acting within the atom were better understood, scientists continued to move toward formulating more complete theories of atomic structure.
There were still questions to answer and challenges to overcome, but Rutherford's findings broke new ground in the study of physics and chemistry in the atomic age.
Impact and acceptance of Rutherford's atomic model
Reception by the scientific community
The atomic model proposed by Rutherford had a significant impact on the scientific community of the time. His theory challenged previous ideas about the structure of the atom and provided a more precise and detailed explanation. Scientists recognized the importance of his findings and the experimental evidence supporting his model.
Numerous additional experiments were carried out to validate and refine this revolutionary theory. Rutherford's discoveries in the gold leaf experiment were especially influential.
The observation that most alpha particles passed through the gold atoms without deflecting, together with the unexpected deflections and bounces of some alpha particles, provided clear evidence for the existence of a dense, positively charged nucleus at the center of the atom.
Advances in the study of the atom and its properties
Rutherford's atomic model allowed significant advances in the understanding of the atom and its properties.
His focus on the atomic nucleus and orbiting electrons laid the foundation for later research in the field of nuclear physics. Scientists were able to analyze and better understand the structure and interaction of subatomic particles. This model also paved the way for the later identification of the proton as the fundamental component of the atomic nucleus.
Subsequent studies on atom stability and electronic distribution led to the development of more complete theories, such as the quantum model, which overcomes the limitations of Rutherford's model. In summary, Rutherford's atomic model had a significant impact on the scientific community of the time and marked a milestone in the study of the atom.
His discoveries and theories laid the foundation for later research in the field of nuclear physics and the understanding of subatomic particles. Despite its limitations, Rutherford's model was a crucial starting point for the advancement of science and the understanding of atomic structure.
- Reception by the scientific community
- Advances in the study of the atom and its properties

